We use our extensive expertise in mucosal immunology and microbiology to develop novel vaccine and drugs that will impact patients in the UK and globally. Our translational research scope ranges from discovery science to clinical trials
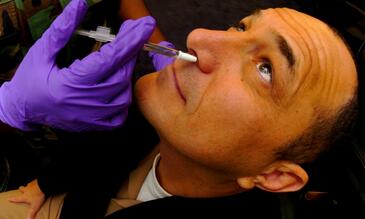
We have developed a method, unique in the world, for inoculating humans safely with live bacteria in order to establish carriage experimentally and have now tested it in over 600 subjects without adverse effects. Testing new vaccines using this controlled human infection model can be done more quickly and at a fraction of the cost of clinical studies (100 subjects rather than many thousands) and so several vaccines candidates under development can be screened. Samples collected in the model are also being used to identify protective responses specific to certain pneumococcal antigens which will then be developed as novel vaccine candidates. Our Programme of work offers an opportunity for partnership with commercial entities or charities sponsoring particular new vaccines and these funding options will be explored with our current funders, MRC and BMGF.

Using experimental human pneumococcal carriage as a way to test new vaccines against the pneumococcus.
Disease caused by the bacterium Streptococcus pneumoniae(pneumococcus) is the most common cause of preventable death in children and a major cause of death among adults world-wide.The current conjugate vaccine is effective in the prevention of both pneumococcal carriage and disease in young children however, it is not as effective against pneumonia and otitis media (ear infection) and it only prevents infection by a small number of pneumococcal types. As such, there is a need for new vaccines and it has recently been suggested that pneumococcal carriage, rather than disease, could be used as an endpoint in vaccine trials.
LSTM’s Vaccine, Immunology and Translational Research Team (VITal), part of the Department of Clinical Sciences at LSTM and the Respiratory Research at the Royal at the RLUH, has developed an experimental human pneumococcal carriage model over the last six years that can be used to test pneumococcal vaccines in development. Demonstrating a reduction in experimental carriage acquisition would provide proof-of-concept for both individual protection and potential reduction in bacterial transmission. The team recently completed a double blind randomized controlled trial using the current conjugate vaccine, PCV13, and demonstrated that acquisition of experimental pneumococcal carriage was reduced by 78% in vaccinated adults, which is similar to what has been shown in vaccinated children.
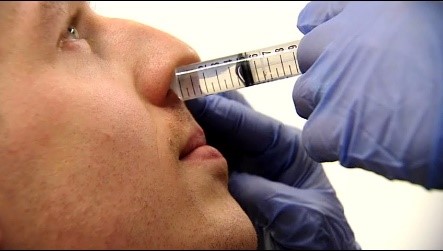
The team is now collaborating with Genocea Biosciences, Inc. a pharmaceutical company based in Cambridge, Massachusetts, to test the ability of their vaccine to protect against carriage. This vaccine, currently named GEN-004, is a novel T cell vaccine that is aimed at preventing carriage of the upper airway by all types of pneumococcus, thereby potentially offering universal protection against pneumococcal disease.

This model has been set up to implement further novel vaccine studies using a similar design to the completed PCV13 study and the trial with Genocea Biosciences. Any companies interested in using the experimental carriage model to test a pneumococcal vaccine should contact Dr Daniela Ferreira.
